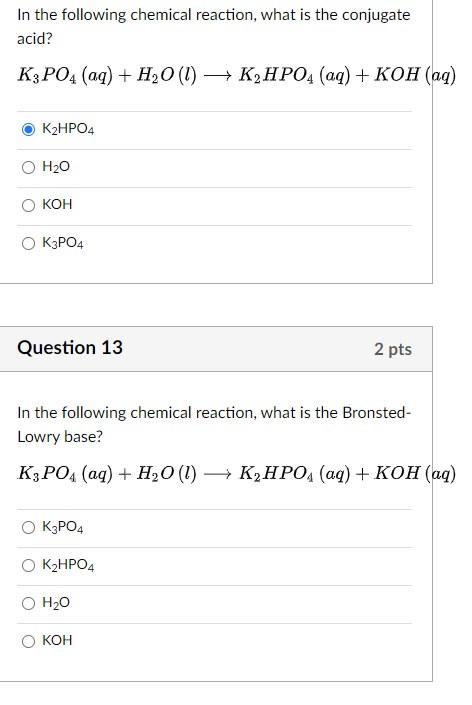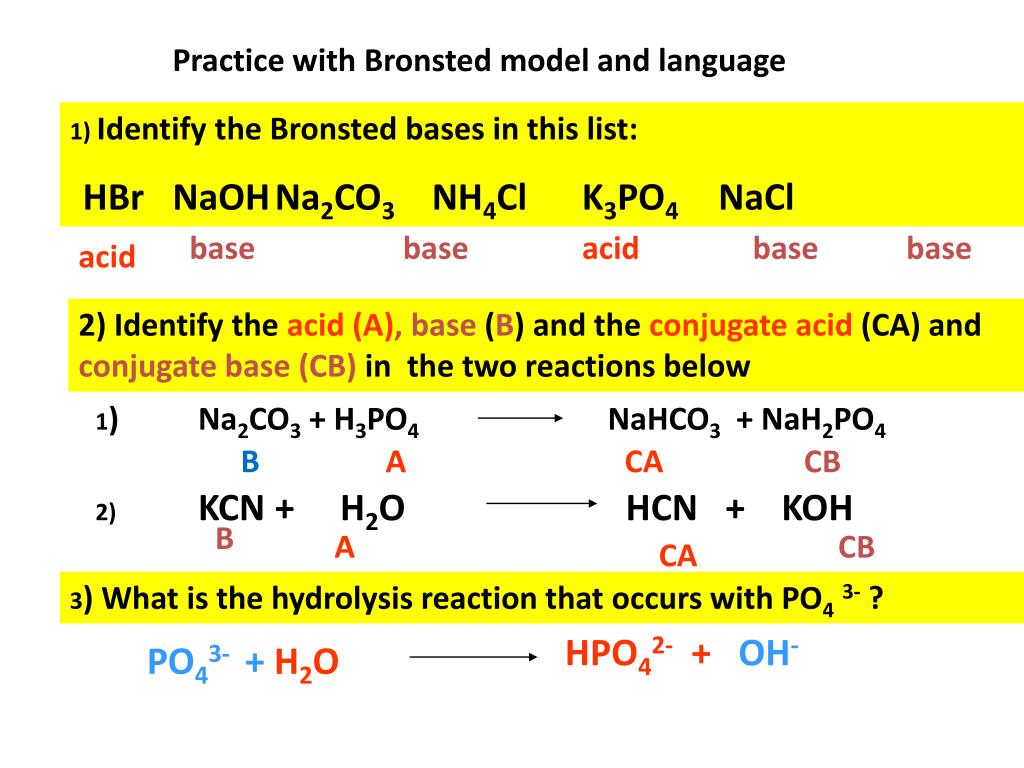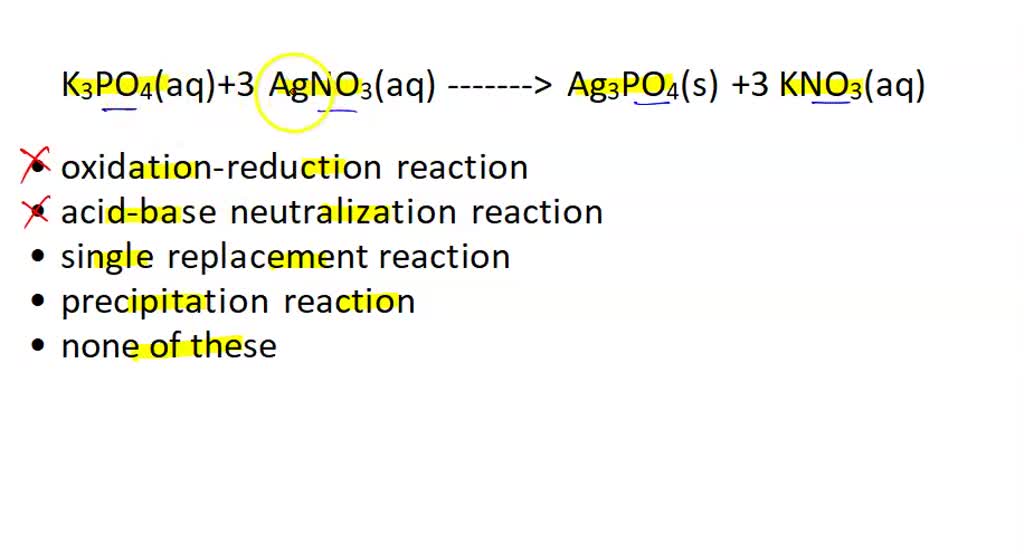
SOLVED: the reaction K3PO4(aq)+3 AgNO3(aq) Ag3PO4(s) +3 KNO3(aq) is best classsfiied as oxidation-reduction reaction acid-base neutralization reaction single replacement reaction precipitation reaction none of these
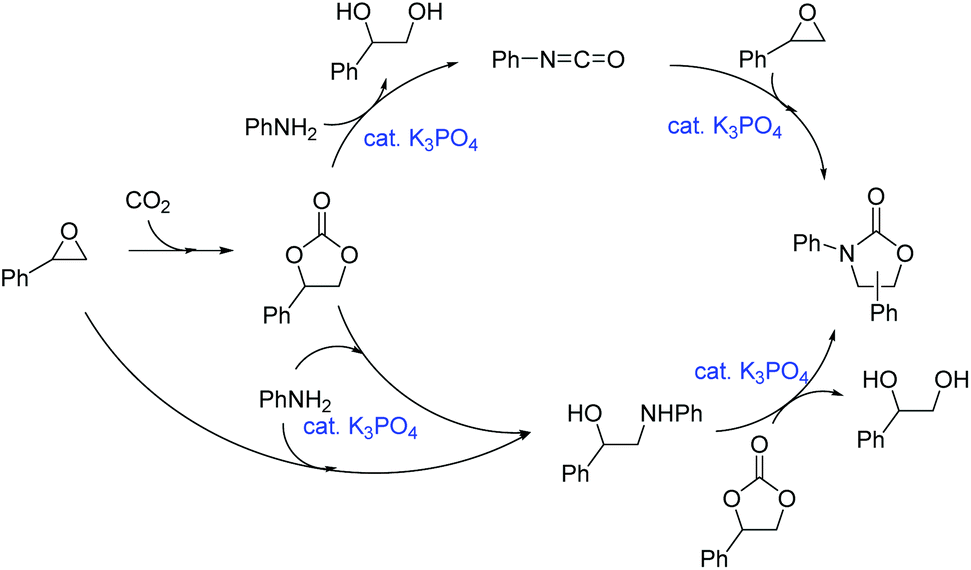
Potassium phosphate-catalyzed one-pot synthesis of 3-aryl-2-oxazolidinones from epoxides, amines, and atmospheric carbon dioxide - Green Chemistry (RSC Publishing) DOI:10.1039/C6GC02934E

The potential of K3PO4, K2CO3, Na3PO4 and Na2CO3 as reusable alkaline catalysts for practical application in biodiesel production - ScienceDirect
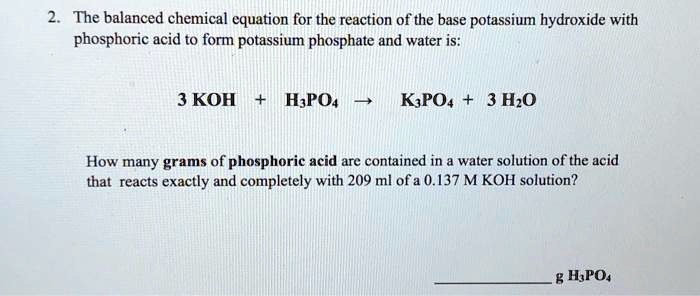
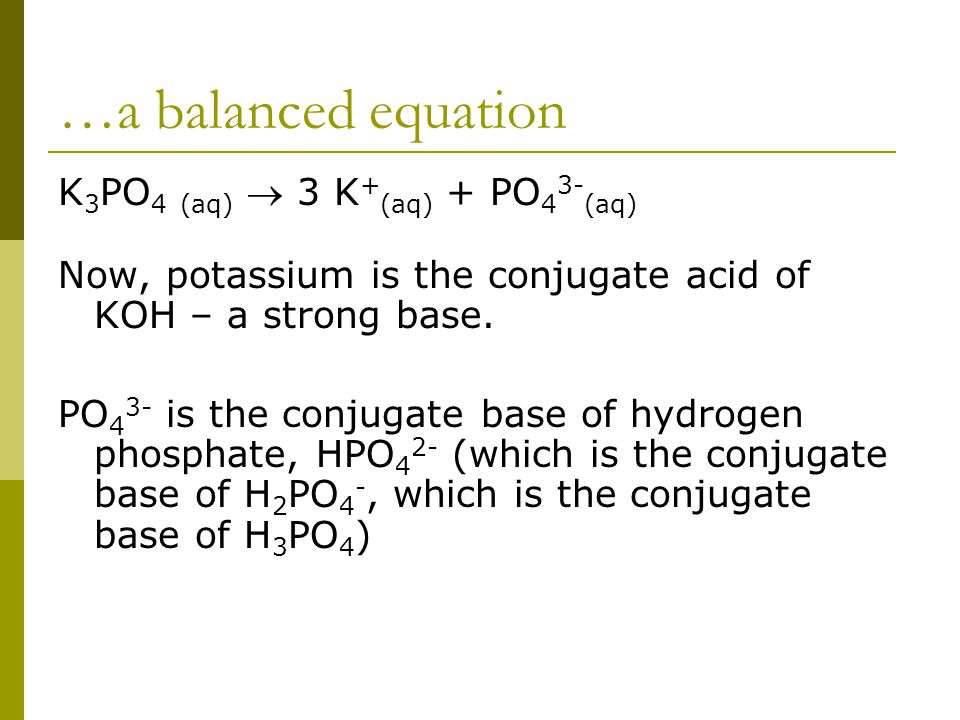
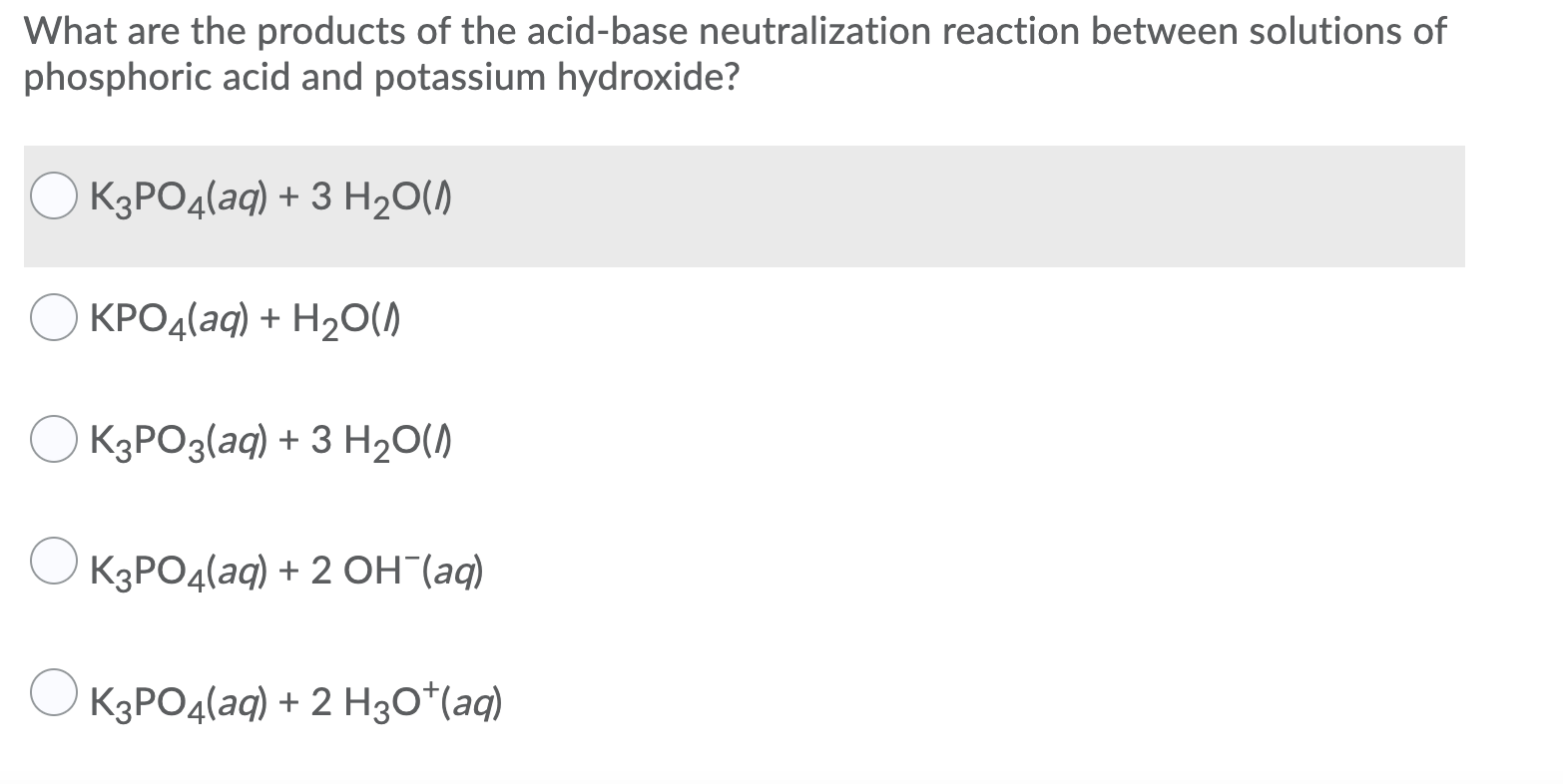
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH HPO4 KaPOs 3 HzO How many grams

K3PO4-Catalyzed Regiospecific Aminobromination of β-Nitrostyrene Derivatives with N-Bromoacetamide as Aminobrominating Agent | The Journal of Organic Chemistry

a) Evolution of the characteristic C=O IR band at 1815 cm -1 during the... | Download Scientific Diagram

EASTCHEM Food Grade 97+% Content(K3PO4,Dry Basis) Tripotassium Phosphate of,CAS NO.:7778-53-2(500g) : Amazon.sg: Industrial & Scientific
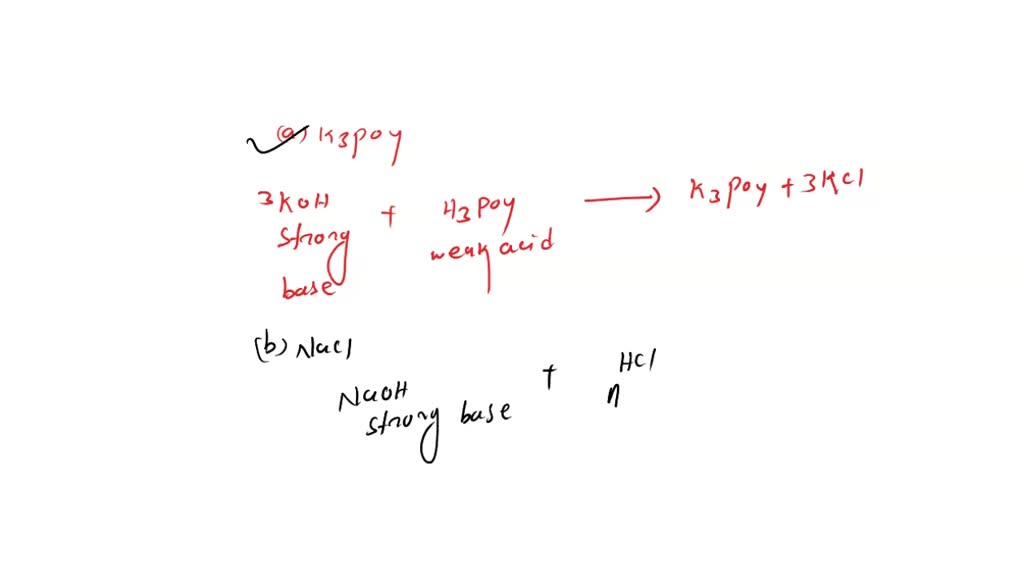
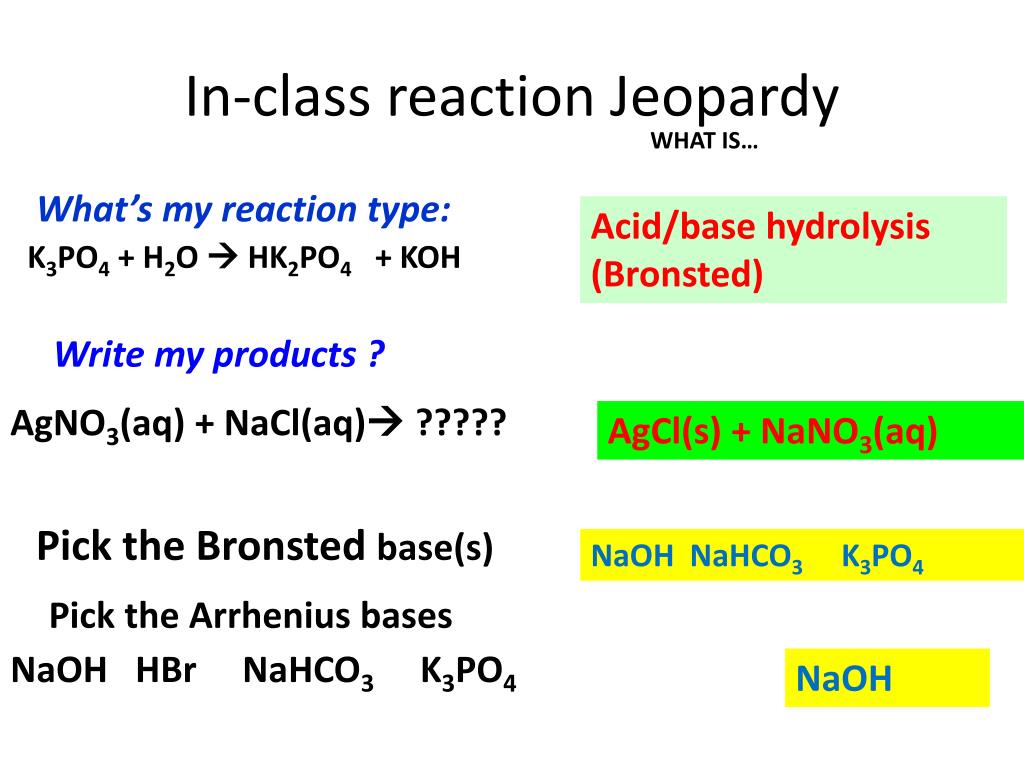
SOLVED: Which of the following salts may be obtained by the reaction of a weak acid with a strong base? a. K3PO4 b. NaCl c. FeCl3 d. LiClO4 e. NH4F

Variation of the nature of the base for the O-methylation of glycerol... | Download Scientific Diagram

Potassium Phosphate as a Solid Base Catalyst for the Catalytic Transfer Hydrogenation of Aldehydes and Ketones | ACS Catalysis