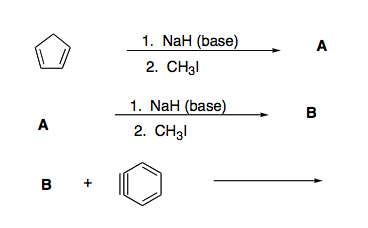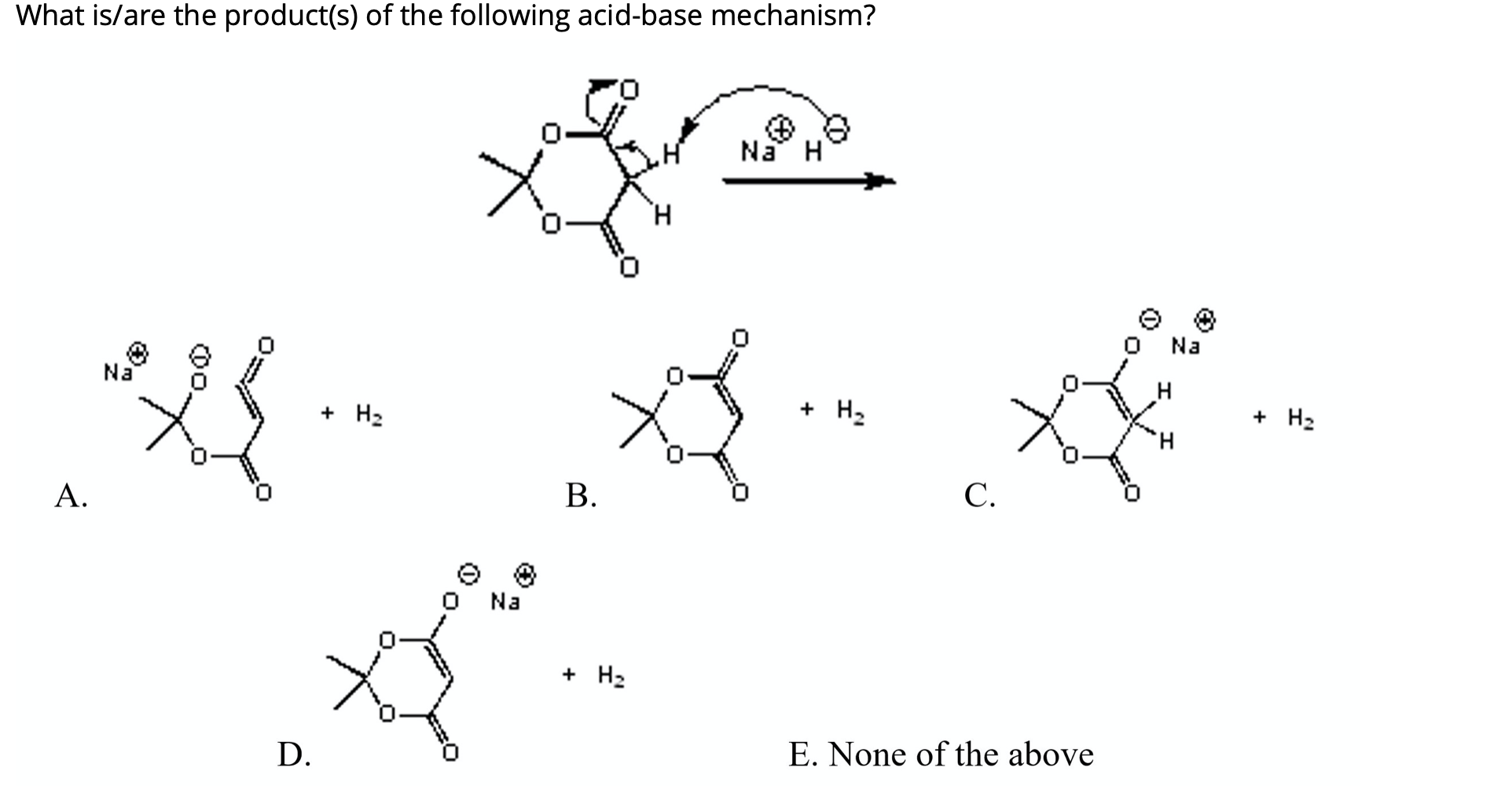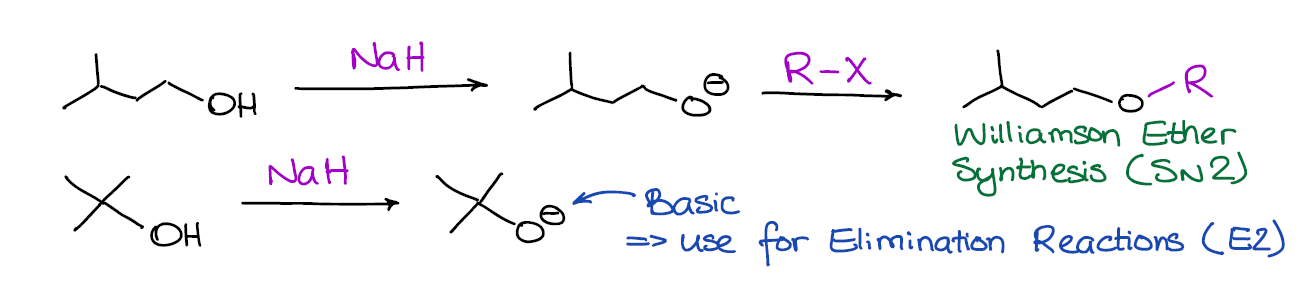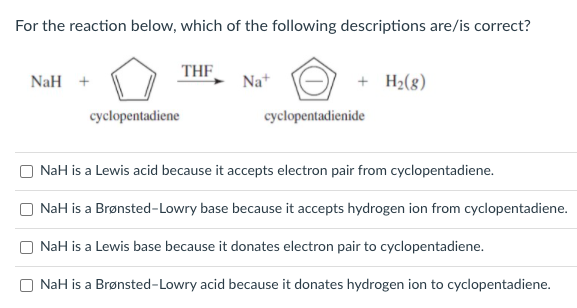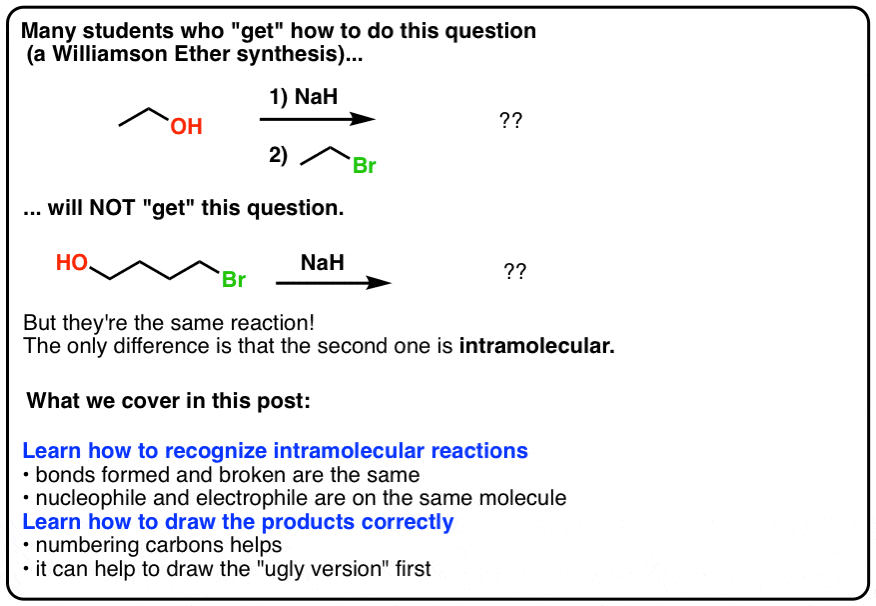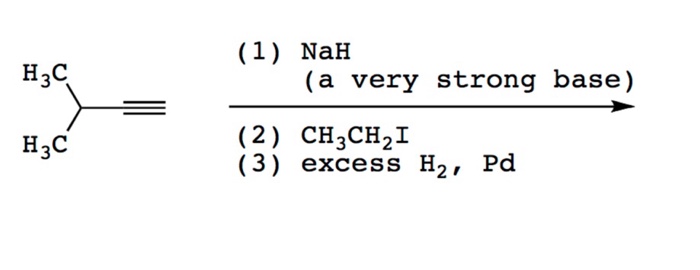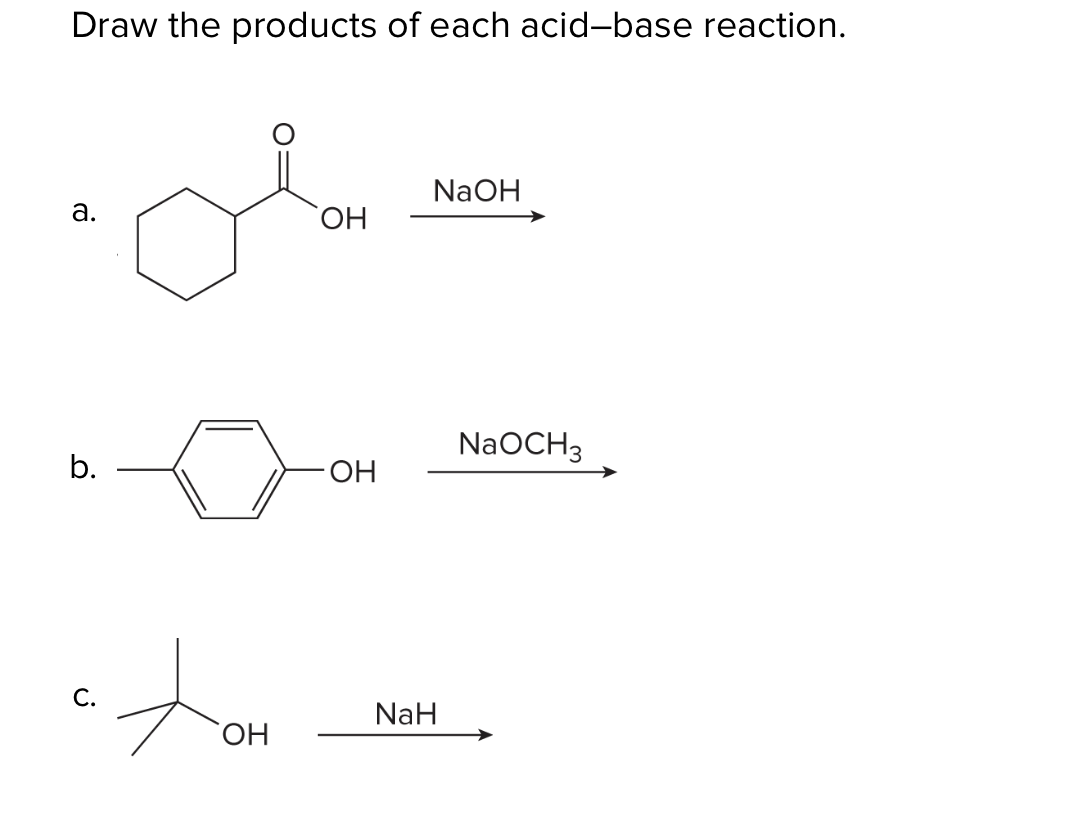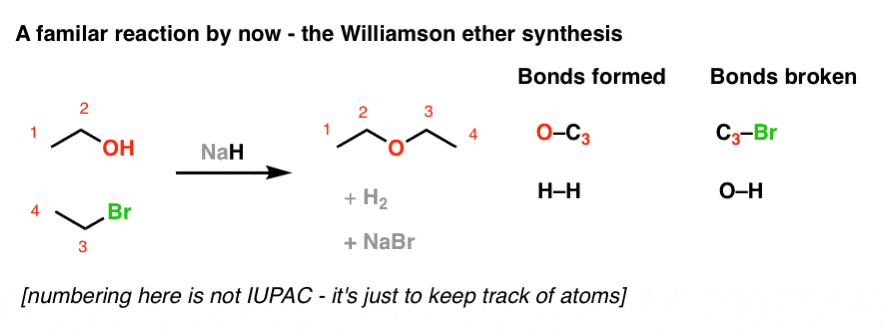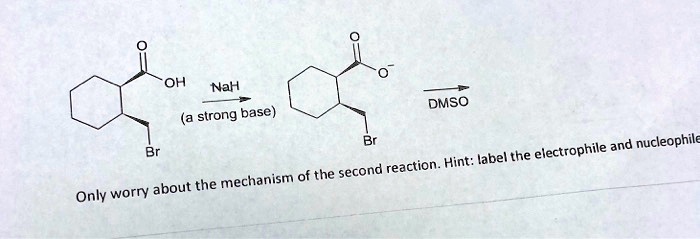
SOLVED: OH NaH DMSO strong base) and nucleophile Hint: label the electrophile second reaction mechanism of the Only worry about the

OneClass: Predict the structures of BOTH bronsted acid base reaction of NaH with typical thiol. What ...

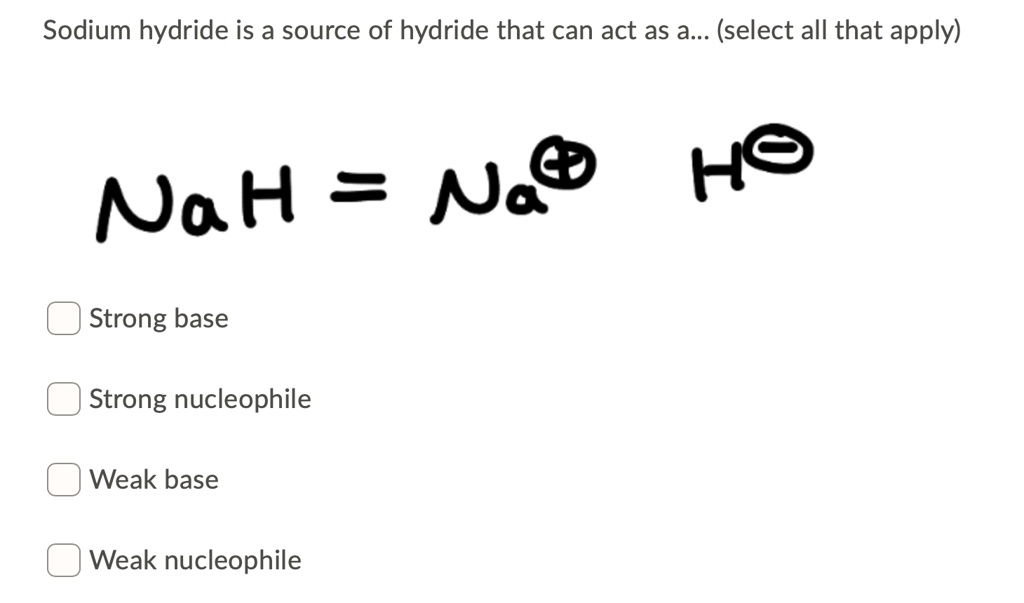
SOLVED: Sodium hydride (NaH) is convenient source Of the hydride anion (H ) the conjugate base of Hz: In contrast t0 other hydride reagents that we will learn about next semester; NaH

The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?

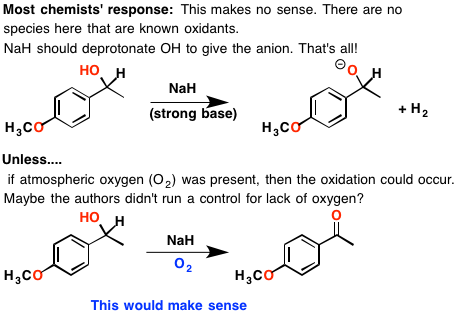
Complications from Dual Roles of Sodium Hydride as a Base and as a Reducing Agent | The Journal of Organic Chemistry
✓ Solved: When 4-chlorobutane-1-thiol is treated with a strong base such as sodium hydride, NaH, tetrahydrothiophene...

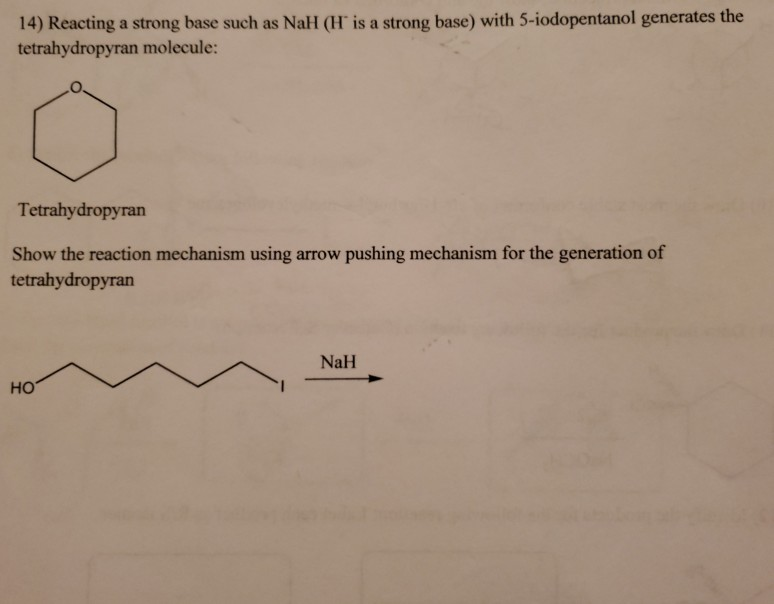
When the halohydrin is treated with NaH, a product of molecular formula C_4H_8O is formed. Draw the structure of the product and indicate its stereochemistry. | Homework.Study.com