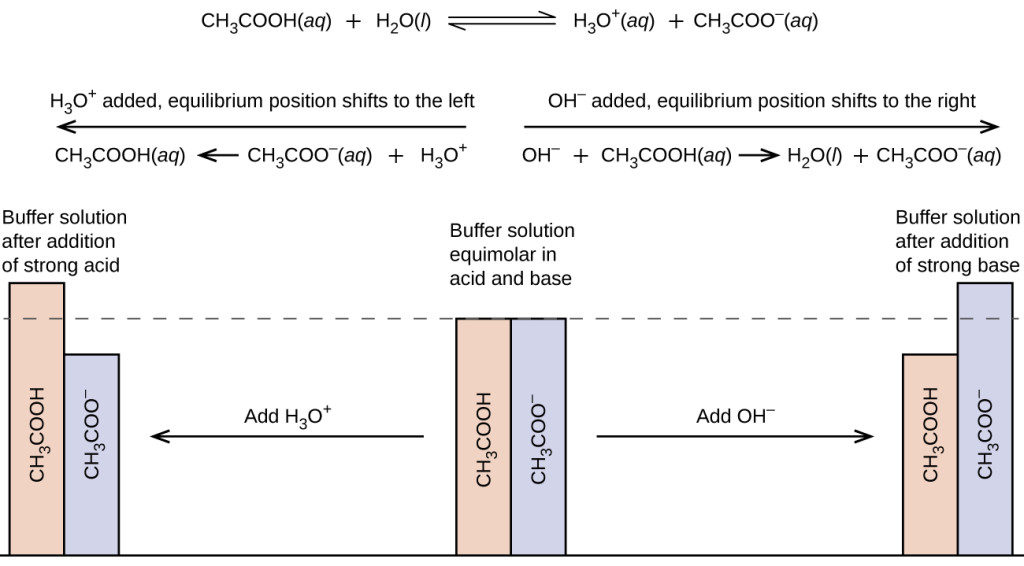
If sodium acetate is a weak acid and does not readily dissociate in water or completely and a strong electrolyte is defined as the oppposite how come the answer is B and

You have 250mL of a 0.56M solution of sodium acetate. How many mL of 0.50M acetic acid should be added to make a buffer of pH 4.40? | Homework.Study.com

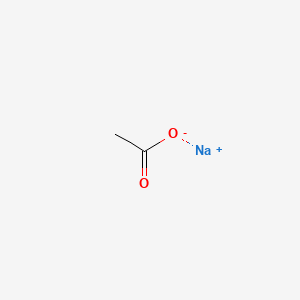
SOLVED: Sodium acetate (NaC2H3O2) is a basic salt. When sodium acetate is dissolved in water, it dissociates into its component ions. This reaction goes to completion, as indicated by the one-way arrow
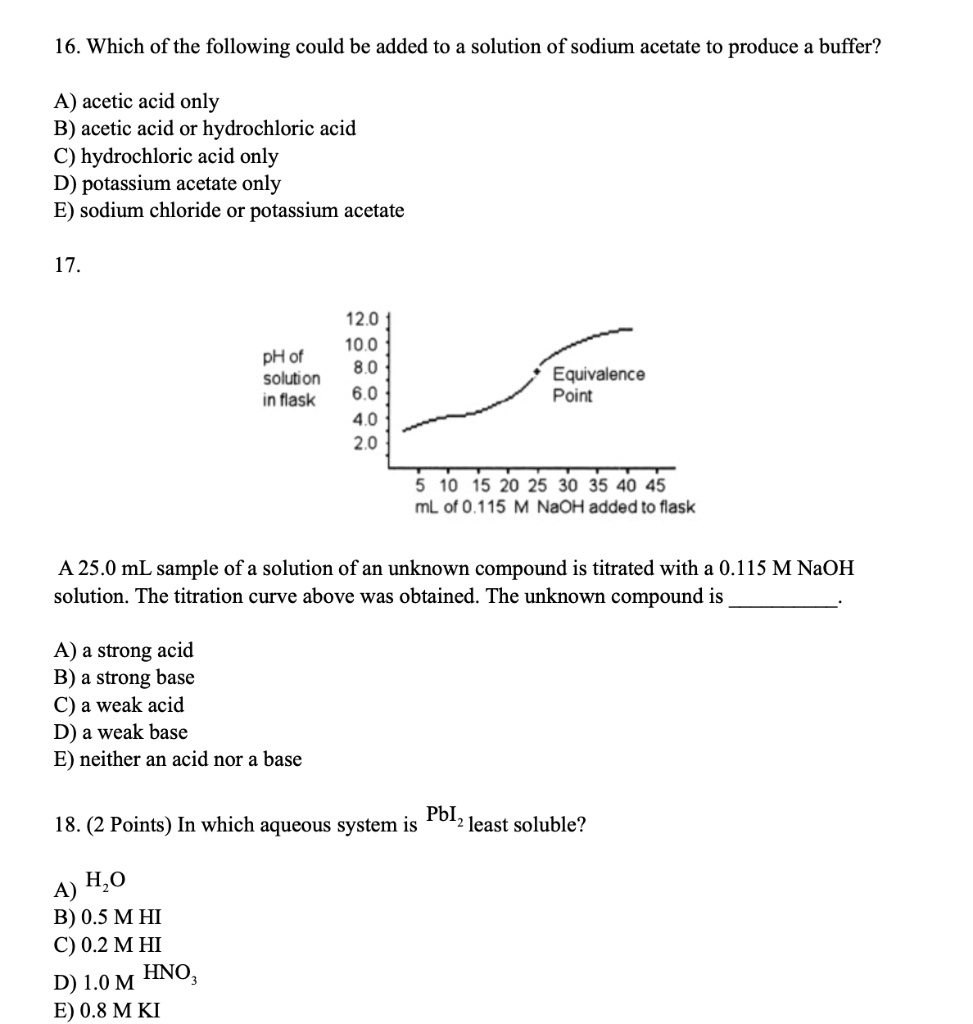
SOLVED: 16. Which of the following could be added to a solution of sodium acetate to produce a buffer? A) acetic acid only B) acetic acid or hydrochloric acid C) hydrochloric acid
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora










![BS005] 3M Sodium Acetate, pH 5.2 | Biosolution BS005] 3M Sodium Acetate, pH 5.2 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2016/02/BS005-Sodium-Acetate-Solution.jpg)